Photocatalytic mechanisms of indoleamine destruction by the quinalphos metabolite 2-hydroxyquinoxaline: a study on melatonin and its precursors serotonin and N-acetylserotonin.
Andreas Behrends, Sonja Riediger, Sascha Grube, Burkhard Poeggeler, Chandana Haldar, Rüdiger Hardeland
文献索引:J. Environ. Sci. Health B 42(6) , 599-606, (2007)
全文:HTML全文
摘要
The redox-active quinalphos main metabolite, 2-hydroxyquinoxaline, is particularly effective under excitation by light. We have studied the photocatalytic destruction of melatonin and its precursors, because the cytoprotective indoleamine has been detected in high quantities in mammalian skin. In photooxidation reactions, in which melatonin, N-acetylserotonin and serotonin are destroyed by 2-hydroxyquinoxaline, the photocatalyst is virtually not consumed. Rates of melatonin and serotonin destruction are not changed by the singlet oxygen quencher 1,4-diazabicyclo-(2,2,2)-octane, indicating that this oxygen species is not involved in the primary reactions, so that the persistence of 2-hydroxyquinoxaline has to be explained by redox cycling. This should imply formation of an organic radical, presumably the quinoxaline-2-oxyl radical, from which 2-hydroxyquinoxaline is regenerated by electron abstraction from indolic radical scavengers. Electron donation by 2-hydroxyquinoxaline is demonstrated by reduction of the 2,2'-azino-bis-(3-ethylbenzthiazolinyl-6-sulfonic acid) cation radical under ultrasound excitation. The compound 2-hydroxyquinoxaline interacts with the specific superoxide anion scavenger Tiron. Formation of oligomeric products from melatonin and serotonin is strongly inhibited by sodium dithionite. Products from photocatalytic indolamine conversion are predominantly dimers and oligomers. No kynuramines were detected in the case of serotonin oxidation, and melatonin's otherwise prevailing oxidation product N(1)-acetyl-N(2)-formyl-5-methoxykynuramine, another cytoprotective metabolite, is only formed in relatively small quantities. The proportion between products from melatonin is changed by 1,4-diazabicyclo-(2,2,2)-octane: singlet oxygen, also formed under the influence of excited 2-hydroxyquinoxaline, only affects secondary reactions.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
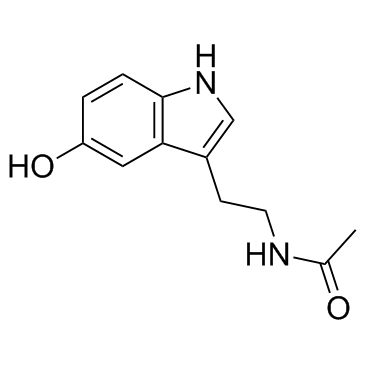 |
N-乙酰-5-羟基色胺
CAS:1210-83-9 |
C12H14N2O2 | |
 |
喹硫磷
CAS:13593-03-8 |
C12H15N2O3PS |
|
Chemical genetics reveals a complex functional ground state ...
2007-05-01 [Nat. Chem. Biol. 3(5) , 268-273, (2007)] |
|
The antioxidant behaviour of melatonin and structural analog...
2012-07-13 [Biochem. Biophys. Res. Commun. 423(4) , 873-7, (2012)] |
|
Clock-Controlled Regulation of the Acute Effects of Norepine...
2015-12-01 [J. Biol. Rhythms 30 , 519-32, (2015)] |
|
An UPLC-MS-based metabolomics investigation on the anti-fati...
2015-02-01 [J. Pharm. Biomed. Anal. 105 , 84-90, (2015)] |
|
Antiplatelet aggregation and endothelial protection of I4, a...
2015-01-01 [Platelets 26 , 342-8, (2015)] |