19F NMR study on the biological Baeyer-Villiger oxidation of acetophenones.
M J Moonen, I M Rietjens, W J van Berkel
文献索引:J. Ind. Microbiol. Biotechnol. 26(1-2) , 35-42, (2001)
全文:HTML全文
摘要
The biological Baeyer-Villiger oxidation of acetophenones was studied by 19F nuclear magnetic resonance (NMR). The 19F NMR method was used to characterise the time-dependent conversion of various fluorinated acetophenones in either whole cells of Pseudomonas fluorescens ACB or in incubations with purified 4'-hydroxyacetophenone monooxygenase (HAPMO). Whole cells of P. fluorescens ACB converted 4'-fluoroacetophenone to 4-fluorophenol and 4'-fluoro-2'-hydroxyacetophenone to 4-fluorocatechol without the accumulation of 4'-fluorophenyl acetates. In contrast to 4-fluorophenol, 4-fluorocatechol was further degraded as evidenced by the formation of stoichiometric amounts of fluoride anion. Purified HAPMO catalysed the strictly NADPH-dependent conversion of fluorinated acetophenones to fluorophenyl acetates. Incubations with HAPMO at pH 6 and 8 showed that the enzymatic Baeyer-Villiger oxidation occurred faster at pH 8 but that the phenyl acetates produced were better stabilised at pH 6. Quantum mechanical characteristics explained why 4'-fluoro-2'-hydroxyphenyl acetate was more sensitive to base-catalysed hydrolysis than 4'-fluorophenyl acetate. All together, 19F NMR proved to be a valid method to evaluate the biological conversion of ring-substituted acetophenones to the corresponding phenyl acetates, which can serve as valuable synthons for further production of industrially relevant chemicals.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
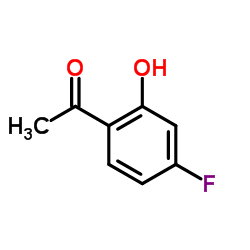 |
4'-氟-2'-羟基苯乙酮
CAS:1481-27-2 |
C8H7FO2 |
|
Synthesis, characterization and biological evaluation of nov...
2015-06-01 [J. Enzyme Inhib. Med. Chem. , 1-8, (2014)] |
|
[J. Heterocycl. Chem. 30 , 445, (1993)] |
|
4'-Fluoro-2'-hydroxyacetophenone. Rizal MR and Ng SW.
[Acta Crystallogr. Sect. E Struct. Rep. Online 64(5) , o916, (2008)] |