Antichymotrypsin interaction with chymotrypsin. Intermediates on the way to inhibited complex formation.
Y Luo, Y Zhou, B S Cooperman
文献索引:J. Biol. Chem. 274(25) , 17733-41, (1999)
全文:HTML全文
摘要
Serpins form enzymatically inactive covalent complexes (designated E*I*) with their target proteinases, corresponding most likely to the acyl enzyme that resembles the normal intermediate in substrate turnover. Formation of E*I* involves large changes in the conformation of the reactive center loop (residues P17 to P9') and of the serpin molecule in general. The "hinge" region of the reactive center loop, including residues P10-P14, shows facile movement in and out of beta-sheet A, and this movement appears to be crucial in determining whether E*I* is formed (the inhibitor pathway) or whether I is rapidly hydrolyzed to I* (the substrate pathway). Here, we report stopped-flow and rapid quench studies investigating the pH dependence of the conversion of the alpha1-antichymotrypsin.alpha-chymotrypsin encounter complex, E.I, to E*I*. These studies utilize fluorescent derivatives of cysteine variants of alpha1-antichymotrypsin at the P11 and P13 residues. Our results demonstrate three identifiable intermediates, EIa, EIb, and EIc, between E.I and E*I* and permit informed speculation regarding the nature of these intermediates. Partitioning between inhibitor and substrate pathways occurs late in the process of E*I* formation, most likely from a species occurring between EIc and E*I*.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
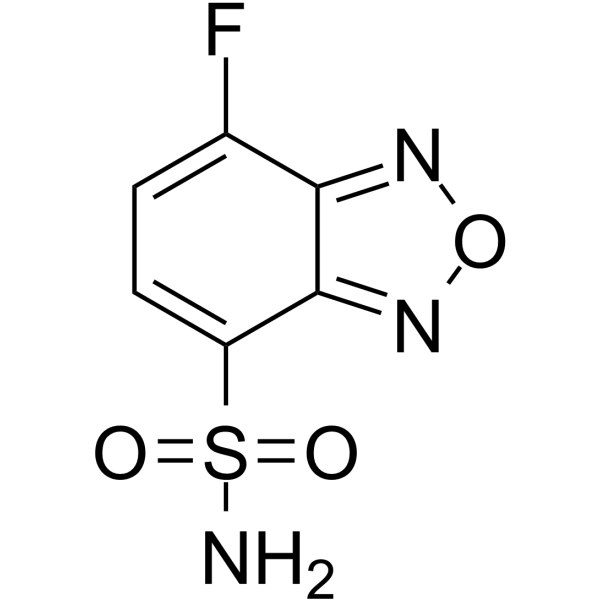 |
7-氟苯呋咱-4-硫氨
CAS:91366-65-3 |
C6H4FN3O3S |
|
Quantification of glutathione in plasma samples by HPLC usin...
2012-02-01 [J. Chromatogr. Sci. 50 , 119-122, (2012)] |
|
Plasma thiols levels in Alzheimer's disease mice under diet-...
2015-01-01 [J. Alzheimers Dis. 44(4) , 1323-31, (2015)] |
|
Allosteric inhibition of PTP1B activity by selective modific...
2005-05-31 [Biochemistry 44(21) , 7704-12, (2005)] |
|
Characterization of cysteine residues of glutathione S-trans...
1992-10-15 [Biochem. Biophys. Res. Commun. 188(1) , 424-32, (1992)] |
|
Removal of the fluorescent 4-(aminosulfonyl)-2,1,3-benzoxadi...
1998-03-06 [J. Chromatogr. A. 798(1-2) , 47-54, (1998)] |