| 结构式 | 名称/CAS号 | 全部文献 |
|---|---|---|
 |
溴乙酸叔丁酯
CAS:5292-43-3 |
|
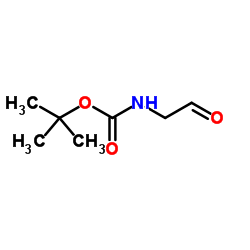 |
N-Boc-2-氨基乙醛
CAS:89711-08-0 |
| 结构式 | 名称/CAS号 | 全部文献 |
|---|---|---|
 |
溴乙酸叔丁酯
CAS:5292-43-3 |
|
 |
N-Boc-2-氨基乙醛
CAS:89711-08-0 |