A naturalistic evaluation and audit database of agomelatine: clinical outcome at 12 weeks.
A Sparshatt, R H McAllister Williams, D S Baldwin, P M Haddad, S Bazire, E Weston, P Taylor, D Taylor
文献索引:Acta Psychiatr. Scand. 128(3) , 203-11, (2013)
全文:HTML全文
摘要
To determine the effectiveness of agomelatine in routine clinical practice and explore factors associated with response and continuation.Consecutive patients prescribed agomelatine in participating psychiatric services were included. Patient demographic and outcome data were collected at treatment initiation and then at weeks 4, 8 and 12. Outcomes were analysed with respect to clinical and demographic factors.A total of 110 patients from nine NHS trusts were followed through 12 weeks of treatment. Agomelatine was largely used in difficult-to-treat or refractory patients: 83 (75%) had failed to respond to, or relapsed on, prior antidepressants. There were high rates of physical (54.5%) and psychiatric (50.0%) comorbidity. At 12 weeks of treatment, 68 (62%) continued agomelatine treatment. Overall, 69 subjects (62.7%) improved by at least one point of the Clinical Global Impression (severity) scale. Of 42 who discontinued, 23 (56%) discontinued because of lack of efficacy and 10 (24%) due to an adverse event. Of all variables examined, only a history of more than five episodes of depression significantly predicted discontinuation of treatment (OR continuation - 0.36, 95% CI 0.14, 0.95).Agomelatine was effective and generally well tolerated in a cohort of difficult-to-treat patients in clinical practice.© 2012 John Wiley & Sons A/S. Published by John Wiley & Sons Ltd.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
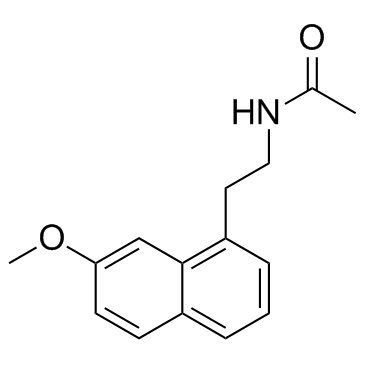 |
阿戈美拉汀
CAS:138112-76-2 |
C15H17NO2 |
|
[The activity of glutathione antioxidant system at melaksen ...
2013-01-01 [Biomed. Khim. 59(5) , 541-9, (2013)] |
|
[Tachycardia and precordial pain with agomelatine: a case re...
2013-01-01 [Therapie. 68(5) , 324-5, (2013)] |
|
Agomelatine, melatonin and depressive disorder.
2013-04-01 [Expert Opin. Investig. Drugs 22(4) , 407-10, (2013)] |
|
Agomelatine may improve REM sleep behavior disorder symptoms...
2012-10-01 [J. Clin. Psychopharmacol. 32(5) , 732-4, (2012)] |
|
Melatonin analogue agomelatine reduces rabbit's intraocular ...
2013-02-15 [Eur. J. Pharmacol. 701(1-3) , 213-7, (2013)] |