| 结构式 | 名称/CAS号 | 全部文献 |
|---|---|---|
 |
辅酶I
CAS:53-84-9 |
|
 |
头孢噻呋
CAS:80370-57-6 |
|
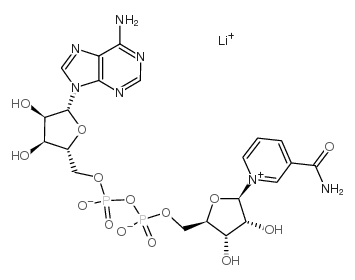 |
Β-菸鹼醯胺腺嘌呤二核苷酸
CAS:64417-72-7 |