| 结构式 | 名称/CAS号 | 全部文献 |
|---|---|---|
 |
嘌呤核苷磷酸化酶
CAS:9030-21-1 |
|
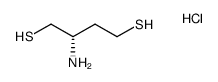 |
(S)-2-氨基丁烷-1,4-二硫醇盐酸盐
CAS:1363376-98-0 |