| 结构式 | 名称/CAS号 | 全部文献 |
|---|---|---|
 |
羧肽酶A 来源于牛胰腺
CAS:11075-17-5 |
|
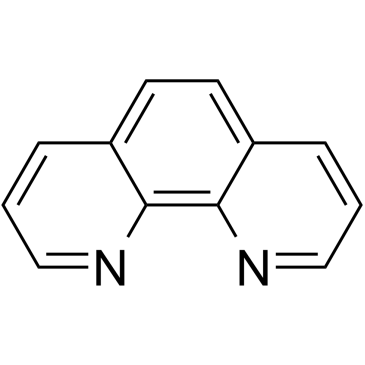 |
1,10-菲罗啉
CAS:66-71-7 |
| 结构式 | 名称/CAS号 | 全部文献 |
|---|---|---|
 |
羧肽酶A 来源于牛胰腺
CAS:11075-17-5 |
|
 |
1,10-菲罗啉
CAS:66-71-7 |