Cytoplasmic dynein regulates the subcellular distribution of mitochondria by controlling the recruitment of the fission factor dynamin-related protein-1.
Aniko Varadi, Linda I Johnson-Cadwell, Vincenzo Cirulli, Yisang Yoon, Victoria J Allan, Guy A Rutter
文献索引:J. Cell Sci. 117(Pt 19) , 4389-400, (2004)
全文:HTML全文
摘要
While the subcellular organisation of mitochondria is likely to influence many aspects of cell physiology, its molecular control is poorly understood. Here, we have investigated the role of the retrograde motor protein complex, dynein-dynactin, in mitochondrial localisation and morphology. Disruption of dynein function, achieved in HeLa cells either by over-expressing the dynactin subunit, dynamitin (p50), or by microinjection of an anti-dynein intermediate chain antibody, resulted in (a) the redistribution of mitochondria to the nuclear periphery, and (b) the formation of long and highly branched mitochondrial structures. Suggesting that an alteration in the balance between mitochondrial fission and fusion may be involved in both of these changes, overexpression of p50 induced the translocation of the fission factor dynamin-related protein (Drp1) from mitochondrial membranes to the cytosol and microsomes. Moreover, a dominant-negative-acting form of Drp1 mimicked the effects of p50 on mitochondrial morphology, while wild-type Drp1 almost completely restored normal mitochondrial distribution in p50 over-expressing cells. Thus, the dynein/dynactin complex plays an unexpected role in the regulation of mitochondrial morphology in living cells, by controlling the recruitment of Drp1 to these organelles.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
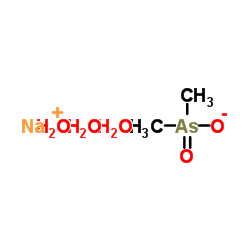 |
二甲砷酸钠三水合物
CAS:6131-99-3 |
C2H12AsNaO5 |
|
MWCNTs of different physicochemical properties cause similar...
2015-04-01 [Toxicol. Appl. Pharmacol. 284(1) , 16-32, (2015)] |
|
Biocompatibility of tungsten disulfide inorganic nanotubes a...
2015-03-01 [Tissue Eng. Part A 21(5-6) , 1013-23, (2015)] |
|
Investigation of commercial sodium cacodylate trihydrate: pe...
2008-01-01 [Acta Crystallogr. C 64(Pt 1) , m13-6, (2008)] |
|
Highly efficient endosomal labeling of progenitor and stem c...
2003-08-01 [Blood 102(3) , 867-72, (2003)] |
|
Tenascin-C promotes microvascular cell migration and phospho...
2002-05-01 [Cancer Res. 62(9) , 2660-8, (2002)] |