| 结构式 | 名称/CAS号 | 全部文献 |
|---|---|---|
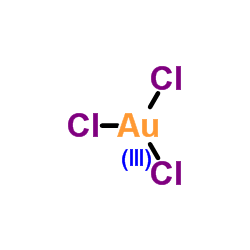 |
三氯化金
CAS:13453-07-1 |
|
 |
氯化亚金(I)
CAS:10294-29-8 |
| 结构式 | 名称/CAS号 | 全部文献 |
|---|---|---|
 |
三氯化金
CAS:13453-07-1 |
|
 |
氯化亚金(I)
CAS:10294-29-8 |