| 结构式 | 名称/CAS号 | 全部文献 |
|---|---|---|
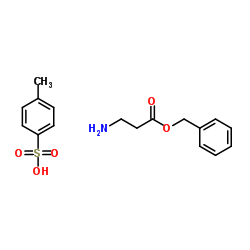 |
beta-丙氨酸苄酯对甲苯磺酸盐
CAS:27019-47-2 |
| 结构式 | 名称/CAS号 | 全部文献 |
|---|---|---|
 |
beta-丙氨酸苄酯对甲苯磺酸盐
CAS:27019-47-2 |