Choline-glycine betaine pathway confers a high level of osmotic tolerance in Escherichia coli.
B Landfald, A R Strøm
文献索引:J. Bacteriol. 165(3) , 849-55, (1986)
全文:HTML全文
摘要
Glycine betaine and its precursors choline and glycine betaine aldehyde have been found to confer a high level of osmotic tolerance when added exogenously to cultures of Escherichia coli at an inhibitory osmotic strength. In this paper, the following findings are described. Choline works as an osmoprotectant only under aerobic conditions, whereas glycine betaine aldehyde and glycine betaine function both aerobically and anaerobically. No endogenous glycine betaine accumulation was detectable in osmotically stressed cells grown in the absence of the osmoprotectant itself or the precursors. A membrane-bound, O2-dependent, and electron transfer-linked dehydrogenase was found which oxidized choline to glycine betaine aldehyde and aldehyde to glycine betaine at nearly the same rate. It displayed Michaelis-Menten kinetics; the apparent Km values for choline and glycine betaine aldehyde were 1.5 and 1.6 mM, respectively. Also, a soluble, NAD-dependent dehydrogenase oxidized glycine betaine aldehyde. It displayed Michaelis-Menten kinetics; the apparent Km values for the aldehyde, NAD, and NADP were 0.13, 0.06, and 0.5 mM, respectively. The choline-glycine betaine pathway was osmotically regulated, i.e., full enzymic activities were found only in cells grown aerobically in choline-containing medium at an elevated osmotic strength. Chloramphenicol inhibited the formation of the pathway in osmotically stressed cells.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
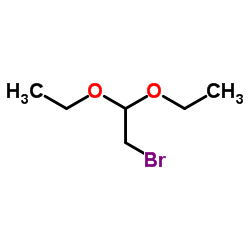 |
2-溴-1,1-二乙氧基乙烷
CAS:2032-35-1 |
C6H13BrO2 |
|
Synthesis and photovoltaic properties of efficient organic d...
2007-05-11 [J. Org. Chem. 72 , 3652, (2007)] |
|
Synthesis, docking, and in vitro activity of thiosemicarbazo...
2006-06-01 [Bioorg. Med. Chem. 14(11) , 3749-57, (2006)] |
|
[Tetrahedron Asymmetry 18 , 557, (2007)] |
|
Synthesis of graft copolymers based on selective living cati...
[J. Polym. Sci. A Polym. Chem. 50(18) , 3703-3709, (2012)] |