Preparation and enantioseparation of a mixed selector chiral stationary phase derived from benzoylated tartaric acid and 1,2-diphenylethylenediamine.
Wen-Juan Wei, Hui-Wen Deng, Wei Chen, Zheng-Wu Bai, Shi-Rong Li
文献索引:Chirality 22(6) , 604-11, (2010)
全文:HTML全文
摘要
L-Dibenzoyl tartaric acid was mono-esterified with benzyl alcohol, and then chlorinated with SOCl(2) to give (2S,3S)-1-(benzyloxy)-4-chloro-1,4-dioxobutane-2,3-diyl dibenzoate (Selector 1). (1R,2R)-1,2-Diphenylethylenediamine was mono-functionalized with phenyl isocyanate and phenylene diisocyanate in sequence to give (1R,2R)-1,2-diphenyl-2-(3-phenylureido)ethyl 4- isocyanatophenylurea (Selector 2). Two brush-type chiral stationary phases (CSPs) of single selector were prepared by separately immobilizing selectors 1 and 2 on aminated silica gel. Selectors 1 and 2 were simultaneously immobilized on aminated silica gel to give a mixed selector CSP. The enantioseparation ability of these CSPs was studied. The CSP of selector 1 has strongest separation ability, while the enantioseparation ability of the mixed selector CSP is relatively lower.(c) 2009 Wiley-Liss, Inc.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
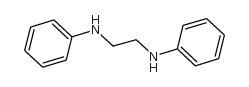 |
N,N'-二苯基乙二胺
CAS:150-61-8 |
C14H16N2 |
|
Role of dermal exposure in systemic intake of methylenediphe...
2015-02-03 [Toxicol. Lett. 232(3) , 595-600, (2015)] |
|
Reversible and irreversible interactions of a cisplatin anal...
1994-01-01 [Drug Metab. Dispos. 22(3) , 419-27, (1994)] |
|
Synthesis of dendritic stationary phases with surface-bonded...
2008-07-01 [Chirality 20(7) , 846-55, (2008)] |
|
Synthesis of polymer-type chiral stationary phases and their...
2007-02-01 [Chirality 19(2) , 129-40, (2007)] |
|
Spectrofluorometric determination of catechins with 1,2-diph...
2002-08-01 [Anal. Sci. 18(8) , 951-3, (2002)] |