| 结构式 | 名称/CAS号 | 全部文献 |
|---|---|---|
 |
4-羟乙基哌嗪乙磺酸
CAS:7365-45-9 |
|
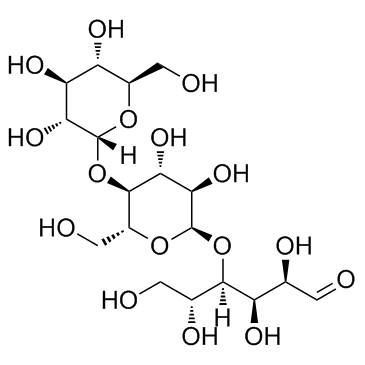 |
麦芽三糖
CAS:1109-28-0 |
|
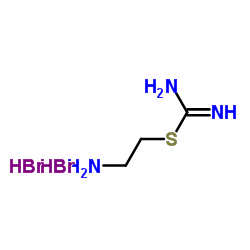 |
S-(2-氨乙基)异硫脲溴鎓氢溴酸盐
CAS:56-10-0 |