Preparation and characterization of conjugates of (modified) human serum albumin and liposomes: drug carriers with an intrinsic anti-HIV activity.
J A Kamps, P J Swart, H W Morselt, R Pauwels, M P De Béthune, E De Clercq, D K Meijer, G L Scherphof
文献索引:Biochim. Biophys. Acta 1278(2) , 183-90, (1996)
全文:HTML全文
摘要
Human serum albumin (HSA) derivatized with cis-aconitic anhydride (Aco-HSA) that was earlier shown to inhibit replication of human immunodeficiency virus type 1 (HIV-1), was covalently coupled to conventional liposomes, consisting of phosphatidylcholine, cholesterol and maleimido-4-(p-phenylbutyryl)phosphatidylethanolamine, using the heterobifunctional reagent N-succinimidyl-S-acetylthioacetate (SATA). The amount of HSA that could be coupled to the liposomes depended on derivatization of the HSA and ranged from 64.2 +/- microgram HSA/micromol total lipid for native HSA to 29.5 +/- 2.7 microgram HSA/micromol total lipid for HSA in which 53 of the epsilon amino groups of lysine were derivatized with cis-aconitic anhydride (Aco53-HSA). Incorporation of 3.8 mol% of total lipid of a poly(ethylene glycol) derivative of phosphatidylethanolamine (PEG-PE) in the liposomes resulted in a lower coupling efficiency of Aco-HSA. The elimination and distribution of the liposomal conjugates in rats in vivo was largely dependent on the modification of the HSA coupled to the liposomes. With native HSA-liposomes, more than 70% of the conjugate was still found in the blood plasma 30 min after i.v. injection in rats, while at this time Aco-HSA-liposomes were completely cleared from the circulation. The rapid clearance of conventional Aco-HSA-liposomes was due to a rapid uptake into the liver and could be considerably decreased by incorporating PEG-PE in the liposomal bilayer. After 3 h 60% of Aco-HSA-PEG-liposome conjugates were found in the blood. In an in vitro anti-HIV-1 assay, the 50% inhibitory concentrations (IC50) for Aco39-HSA-liposomes and Aco53-HSA-liposomes expressed as protein weight, were 2.87 microgram/ml and 0.154 microgram/ml, respectively. When PEG-PE was incorporated, the Aco53-HSA-liposomes retained anti HIV-1 activity (IC50:3.13 microgram/ml). The possibility to modulate the residence time in the bloodstream of Aco-HSA-liposomes and the potent anti-HIV-1 activity of these conjugates, may allow the development of an intrinsically active drug carrier system. By incorporating anti HIV-1 drugs such as AZT into such liposomes a drug delivery system can be designed that might act simultaneously on the virus/cell binding by virtue of the coupled Aco-HSA and on the RNA/DNA transcription of the HIV-1 replication cycle through the nucleoside analogue.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
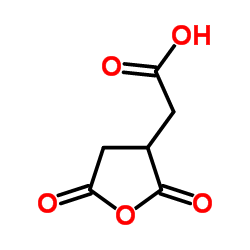 |
顺式乌头酸酐
CAS:6318-55-4 |
C6H4O5 |
|
Enhanced shRNA delivery and ABCG2 silencing by charge-revers...
2015-02-25 [Small 11(8) , 952-62, (2015)] |
|
Development of biodegradable polymeric implants of RGD-modif...
2015-05-01 [Drug Deliv. 22 , 389-99, (2015)] |
|
Acid-responsive PEGylated doxorubicin prodrug nanoparticles ...
2015-12-01 [Colloids Surf. B Biointerfaces 136 , 365-74, (2015)] |
|
Cerebellar Purkinje cells incorporate immunoglobulins and im...
2009-01-01 [J. Neuroinflammation 6 , 31, (2009)] |
|
Lectin-mediated drug targeting: preparation, binding charact...
1998-07-01 [Pharm. Res. 15(7) , 1031-7, (1998)] |