Synergistic TRAIL sensitizers from Barleria alluaudii and Diospyros maritima.
Emily L Whitson, Han Sun, Cheryl L Thomas, Curtis J Henrich, Thomas J Sayers, James B McMahon, Christian Griesinger, Tawnya C McKee
文献索引:J. Nat. Prod. 75(3) , 394-9, (2012)
全文:HTML全文
摘要
Barleria alluaudii and Diospyros maritima were both investigated as part of an ongoing search for synergistic TRAIL (tumor necrosis factor-α-related apoptosis-inducing ligand) sensitizers. As a result of this study, two naphthoquinone epoxides, 2,3-epoxy-2,3-dihydrolapachol (1) and 2,3-epoxy-2,3-dihydro-8-hydroxylapachol (2), both not previously isolated from natural sources, and the known 2-methylanthraquinone (3) were identified from B. alluaudii. Time-dependent density functional theory (TD-DFT) calculations of electronic circular dichroism (ECD) spectra were utilized to establish the absolute configuration of 1 and 2. Additionally, five known naphthoquinone derivatives, maritinone (4), elliptinone (5), plumbagin (6), (+)-cis-isoshinanolone (7), and ethylidene-6,6'-biplumbagin (8), were isolated from D. maritima. Compounds 1, 2, and 4-6 showed varying levels of synergy with TRAIL. Maritinone (4) and elliptinone (5) showed the highest synergistic effect, with more than a 3-fold increase in activity observed with TRAIL than with compound alone.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
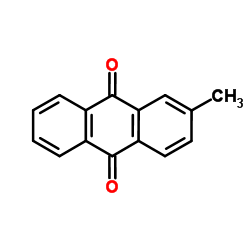 |
2-甲基蒽醌
CAS:84-54-8 |
C15H10O2 |
|
[Chemical constituents of Morinda officinalis How].
1991-11-01 [Zhongguo Zhong Yao Za Zhi 16(11) , 675-6, 703, (1991)] |
|
[Studies on the chemical constituents from stem of Chirita l...
2006-02-01 [Zhongguo Zhong Yao Za Zhi 31(4) , 307-8, (2006)] |
|
PPARγ agonist from Chromolaena odorata.
2012-12-28 [J. Nat. Prod. 75(12) , 2076-81, (2012)] |
|
Isolation, structure elucidation, and cytotoxic evaluation o...
2011-01-28 [J. Nat. Prod. 74(1) , 82-5, (2011)] |
|
Lactones and quinones from the tubers of Sinningia aggregata...
2010-08-27 [J. Nat. Prod. 73(8) , 1434-7, (2010)] |