| 结构式 | 名称/CAS号 | 全部文献 |
|---|---|---|
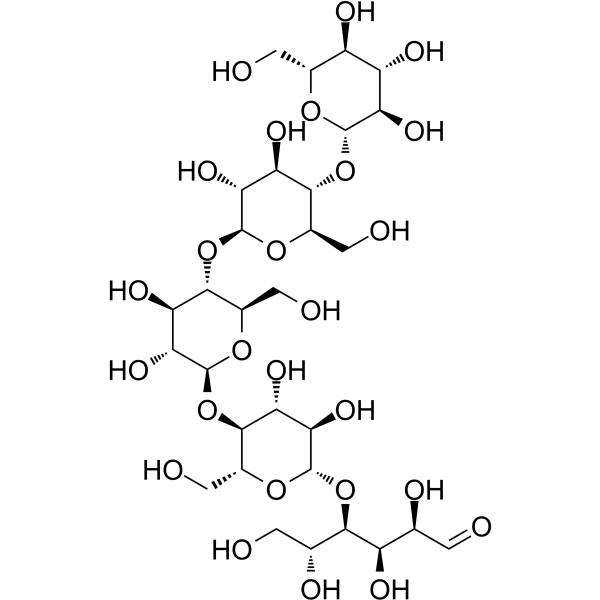 |
纤维五糖
CAS:2240-27-9 |
|
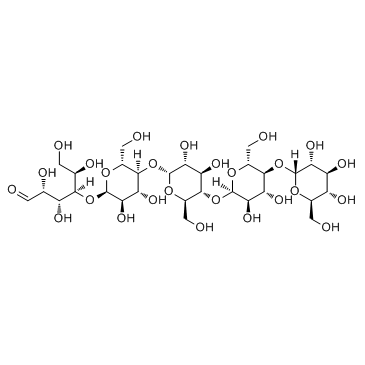 |
麦芽五糖
CAS:34620-76-3 |
| 结构式 | 名称/CAS号 | 全部文献 |
|---|---|---|
 |
纤维五糖
CAS:2240-27-9 |
|
 |
麦芽五糖
CAS:34620-76-3 |