| 结构式 | 名称/CAS号 | 全部文献 |
|---|---|---|
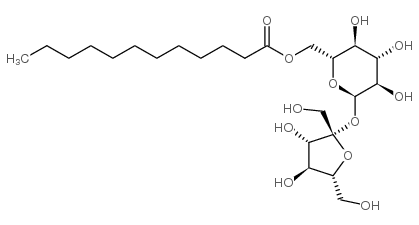 |
蔗糖十二烷酸酯
CAS:25339-99-5 |
|
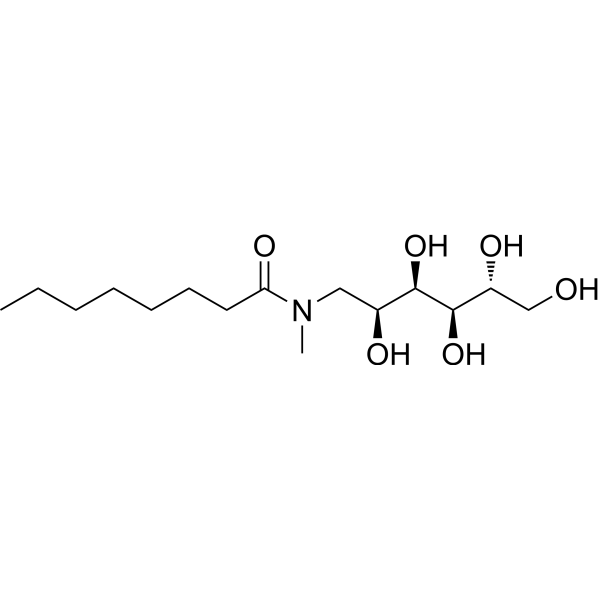 |
N-辛酰基-N-甲基葡糖胺
CAS:85316-98-9 |