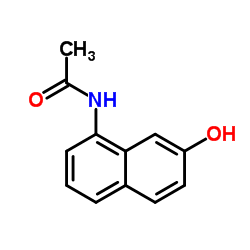
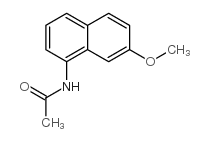
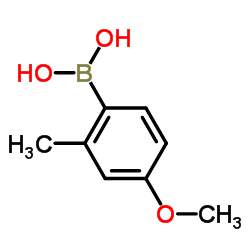
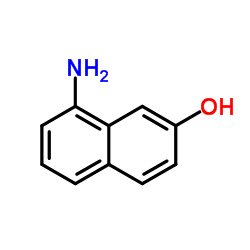
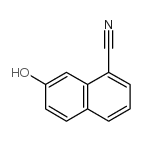
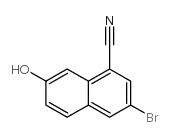
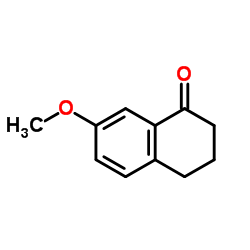
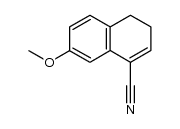
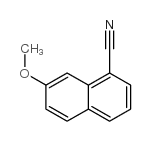
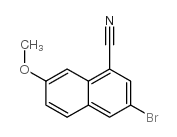
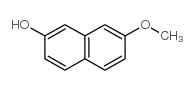
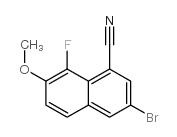
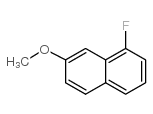
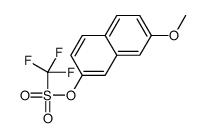
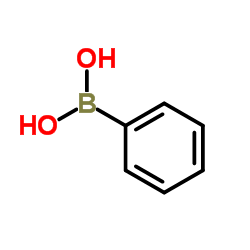
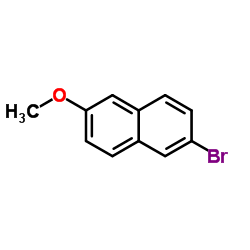
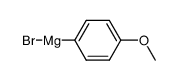
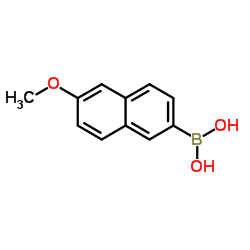
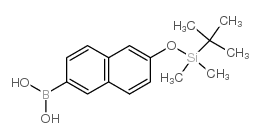
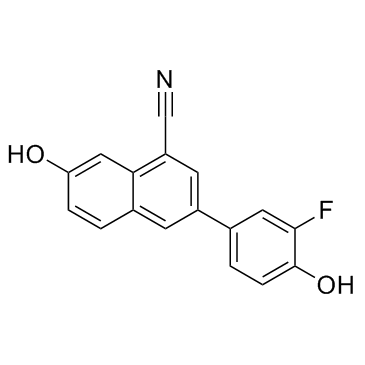
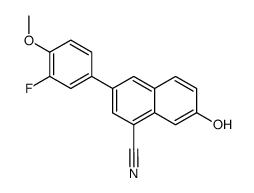
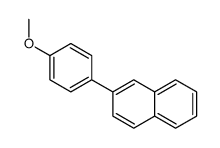
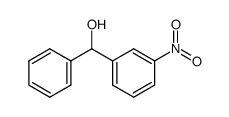
|
~% |
|
~67% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~13% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~85% |
|
~98% |
|
~98% |
|
~80% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~87% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~40% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |