Efficacy of epinastine hydrochloride ophthalmic solution in allergic conjunctivitis by conjunctival cedar pollen allergen challenge.
Hiroshi Fujishima, Yuichi Ohashi, Etsuko Takamura
文献索引:Ann. Allergy Asthma Immunol. 113(4) , 476-81, (2014)
全文:HTML全文
摘要
Epinastine hydrochloride is a selective histamine H1 receptor antagonist that also inhibits IgE receptor-mediated histamine release from mast cells.To show the superiority of epinastine 0.05% ophthalmic solution (epinastine) to placebo ophthalmic solution (placebo) and noninferiority to olopatadine 0.1% ophthalmic solution (olopatadine) for cedar pollen antigen-induced ocular itching and conjunctival hyperemia.The study was conducted in ophthalmologically asymptomatic adult volunteers with seasonal allergic conjunctivitis using a conjunctival allergen challenge test. Subjects were randomized into 3 groups (n = 87) to evaluate superiority to placebo (visits 4 to 6) and 2 groups (n = 86) to evaluate noninferiority to olopatadine (visit 7). At each visit, a single administration of the study medication was instilled at 15 minutes (visit 4), 4 hours (visit 5), 8 hours (visit 6), and 4 hours (visit 7) before the conjunctival allergen challenge test. Ocular itching and conjunctival hyperemia of allergic conjunctivitis were assessed after the conjunctival allergen challenge test.For the primary end point, epinastine showed superiority to placebo for the inhibition of ocular itching and conjunctival hyperemia induced at 4 hours after the dose (equivalent to 4-times-daily dosing). For the secondary end points, epinastine significantly inhibited itching and conjunctival hyperemia induced at 15 minutes and 8 hours after the dose (equivalent to 2-times-daily dosing) compared with placebo. In addition, epinastine demonstrated noninferiority to olopatadine for ocular itching and conjunctival hyperemia. No adverse drug reactions or serious adverse events were reported throughout the study, indicating that epinastine has a good safety profile.Epinastine is effective and safe for the treatment of allergic conjunctivitis.Clinicaltrials.gov identifier NCT01363700.Copyright © 2014 American College of Allergy, Asthma & Immunology. Published by Elsevier Inc. All rights reserved.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
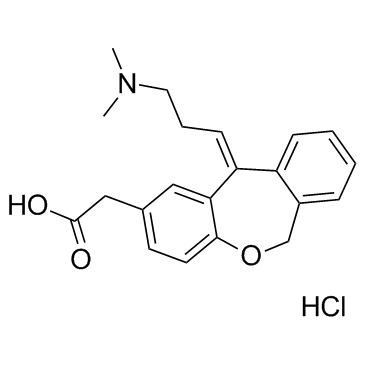 |
盐酸奥洛他定
CAS:140462-76-6 |
C21H24ClNO3 |
|
Topical cyclosporine a 1% for the treatment of chronic ocula...
2014-09-01 [Eye Contact Lens 40(5) , 283-8, (2014)] |
|
Histamine suppresses regulatory T cells mediated by TGF-β in...
2015-04-01 [Exp. Dermatol. 24(4) , 280-4, (2015)] |
|
Chromatographic analysis of olopatadine in hydrophilic inter...
2015-01-01 [J. Chromatogr. Sci. 53 , 680-6, (2015)] |
|
Differential thermodynamic driving force of first- and secon...
2014-09-15 [Biochem. Pharmacol. 91(2) , 231-41, (2014)] |
|
Development and validation of an ultra-performance liquid ch...
2015-03-01 [Biomed. Chromatogr. 29(3) , 465-74, (2015)] |