Remarkable interference with telomeric function by a G-quadruplex selective bisantrene regioisomer
Marco Folini, Claudia Pivetta, Giuseppe Zagotto, Cinzia De Marco, Manlio Palumbo, Nadia Zaffaroni, Claudia Sissi, Marco Folini, Claudia Pivetta, Giuseppe Zagotto, Cinzia De Marco, Manlio Palumbo, Nadia Zaffaroni, Claudia Sissi
文献索引:Biochem. Pharmacol. 79(12) , 1781-90, (2010)
全文:HTML全文
摘要
The use of small molecules able to induce and stabilize selected G-quadruplex arrangements can cause telomerase inhibition and telomere dysfunction in cancer cells, thus providing very selective therapeutic approaches. Effective stabilizers usually comprise a planar aromatic portion to grant effective stacking onto the G-quartet and positively charged side chains to exploit the highly negative charge density on the quadruplex grooves. Since the relative position of these two pharmacophoric moieties is expected to play an important role in DNA folding stabilization, we evaluated a series of anthracene derivatives substituted with one or two 4,5-dihydro-1H-imidazol-2-yl-hydrazonic groups (the bisantrene side chain) at different positions of the aromatic system. Indeed, the various regioisomers showed distinct binding affinities for telomeric G-quadruplex, and the most effective was the 1,5 and 1,7 bis-substituted analogues. On turn, the 1,8 regioisomer was poorly effective. Interestingly, G-quadruplex binding is clearly related to telomerase inhibition in this class of compounds, thus confirming their ability to shift the nucleic acid conformational equilibrium upon binding and consequently produce interference with the telomere processing enzyme. Additionally, the 1,5 regioisomer was shown to inhibit telomerase activity at lower concentrations than those required to reduce tumor cell proliferation. Comparative analysis of drug effects in telomerase-positive and telomerase-negative cancer cells showed consistent cell growth impairment, as a consequence of activation of the senescence pathway, which was mainly attributable to anthracene-mediated telomere dysfunction.Copyright 2010 Elsevier Inc. All rights reserved.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
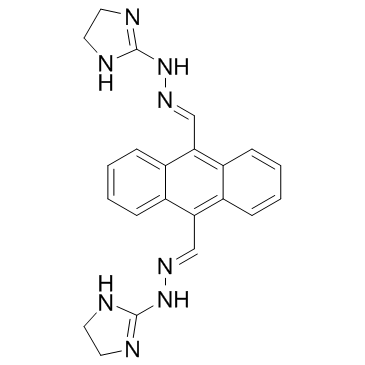 |
比生群
CAS:78186-34-2 |
C22H22N8 |
|
In vitro effects of bisantrene on fresh clonogenic leukemia ...
1990-01-01 [Haematologica 75(6) , 527-31, (1990)] |
|
Phase II trial of Bisantrene for metastatic melanoma: an Ill...
1991-01-01 [Med. Pediatr. Oncol. 19(2) , 126-8, (1991)] |
|
Retroviral transfer of the human MDR1 gene confers resistanc...
2014-04-29 [Clin. Cancer Res. 2(6) , 973-80, (1996)] |
|
Synthesis, DNA-damaging and cytotoxic properties of novel to...
1998-01-20 [Bioorg. Med. Chem. Lett. 8(2) , 121-6, (1998)] |
|
Bisantrene in advanced, hormone-resistant carcinoma of the p...
1990-08-01 [Invest. New Drugs 8(3) , 313-5, (1990)] |