Alzheimer's Abeta40 studied by NMR at low pH reveals that sodium 4,4-dimethyl-4-silapentane-1-sulfonate (DSS) binds and promotes beta-ball oligomerization.
Douglas V Laurents, Paul M Gorman, Meng Guo, Manuel Rico, Avijit Chakrabartty, Marta Bruix
文献索引:J. Biol. Chem. 280(5) , 3675-85, (2005)
全文:HTML全文
摘要
The Alzheimer's Abeta40 peptide forms soluble oligomers that are extremely potent neurotoxins and strongly impede synapses function. In this study the formation and structure of the large, soluble, neurotoxic Abeta40 oligomer called "beta-ball" were characterized by two-dimensional NMR, circular dichroism, fluorescence spectroscopy, hydrogen exchange, and equilibrium sedimentation. In acidic aqueous solution, half the Abeta40 molecules are in the beta-ball state; the remainder are monomeric. The equilibrium between the two states is slow as judged by NMR linewidths and is stable for months. The kinetics of beta-ball formation from monomer are biphasic with tau1 = 7 min and tau2 = 80 min with no transient helix formation. Monomeric Abeta40 is essentially devoid of stable secondary structure, although the central, Leu17-Ala21, and C-terminal, Gly29-Val40, hydrophobic regions show propensity toward adopting extended structure, and residues 22-25 tended to form a turn. We found that sodium 4,4-dimethyl-4-silapentane-1-sulfonate (DSS) binds to the central hydrophobic region of monomeric Abeta40. DSS binds beta-balls more strongly and caused them to double in size. Plausible micelle-like models for the beta-ball structure with and without bound DSS are presented.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
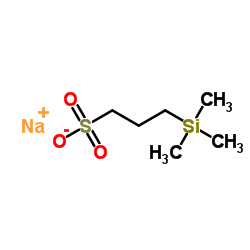 |
3-三甲基硅基-1-丙磺酸钠
CAS:2039-96-5 |
C6H15NaO3SSi |
|
1H-NMR-based profiling of organic components in leachate fro...
2014-09-01 [Environ. Sci. Pollut. Res. Int. 21(17) , 10453-60, (2014)] |
|
Structural elucidation of an asparagine-linked oligosacchari...
2015-09-02 [Carbohydr. Res. 413 , 55-62, (2015)] |
|
Shuttle DNP spectrometer with a two-center magnet.
2010-06-14 [Phys. Chem. Chem. Phys. 12(22) , 5830-40, (2010)] |
|
Synthesis of DOTA-conjugated multivalent cyclic-RGD peptide ...
2007-03-21 [Org. Biomol. Chem. 5(6) , 935-44, (2007)] |
|
Chemoenzymatic synthesis and structural characterization of ...
2014-08-01 [Glycobiology 24(8) , 681-92, (2014)] |