| 结构式 | 名称/CAS号 | 全部文献 |
|---|---|---|
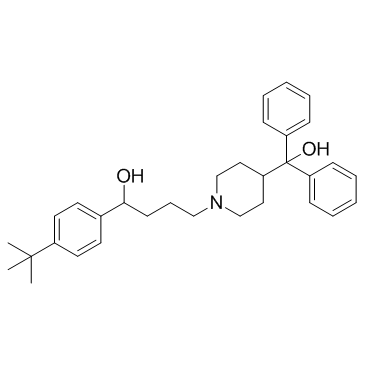 |
特非那定
CAS:50679-08-8 |
|
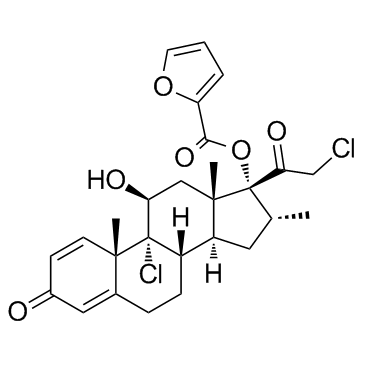 |
糠酸莫米松
CAS:83919-23-7 |
| 结构式 | 名称/CAS号 | 全部文献 |
|---|---|---|
 |
特非那定
CAS:50679-08-8 |
|
 |
糠酸莫米松
CAS:83919-23-7 |