Alpha-keto amide peptides: a synthetic strategy to resin-bound peptide isosteres for protease inhibitor screening on solid support.
Alexandra Papanikos, Morten Meldal
文献索引:J. Comb. Chem. 6(2) , 181-95, (2004)
全文:HTML全文
摘要
A synthetic strategy for the formation of resin-bound internal alpha-keto amide peptides suitable for protease inhibitor screening on solid support is presented. This general approach is based on the incorporation of alpha-keto amide building blocks during solid-phase peptide synthesis (SPPS). Such dipeptidyl building blocks were accessible using the acylcyanophosphorane methodology. The acid-labile alpha-keto carbonyl functionality was protected as a 1,3-dithiolane derivative. This protective group is fully compatible with standard SPPS reaction conditions and can be efficiently removed with N-bromosuccinimide in 10% aqueous acetone. The alpha-keto amide peptides were assembled on SPOCC-1500 resin and were characterized with high-resolution magic angle spinning (HR-MAS) NMR on bead. The methodology was evaluated and tested with a variety of building blocks containing natural and nonnatural amino acid moieties.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
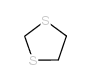 |
1,3-二硫代坊
CAS:4829-04-3 |
C3H6S2 |
|
Cationic cyclization of 2-alkenyl-1,3-dithiolanes: diastereo...
2011-05-06 [J. Org. Chem. 76(9) , 3274-85, (2011)] |
|
gem-Difluorination of 2,2-diaryl-1,3-dithiolanes by Selectfl...
2005-02-07 [Chem. Commun. (Camb.) , 654, (2005)] |
|
Diastereoselective formal total synthesis of (+/-)-triptolid...
2010-08-21 [Chem. Commun. (Camb.) 46(31) , 5778-80, (2010)] |
|
Dithiolane analogs of lignans inhibit interferon-gamma and l...
2000-10-01 [Acta Pharmacol. Sin. 21(10) , 897-904, (2000)] |
|
Synthesis, DNA intercalation and 3D QSAR analysis of cis-2,4...
2009-08-15 [Bioorg. Med. Chem. 17 , 6085-95, (2009)] |