| 结构式 | 名称/CAS号 | 全部文献 |
|---|---|---|
 |
3-氯-1H-吲唑
CAS:29110-74-5 |
|
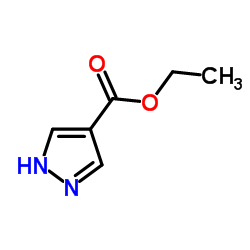 |
4-吡唑甲酸乙酯
CAS:37622-90-5 |
| 结构式 | 名称/CAS号 | 全部文献 |
|---|---|---|
 |
3-氯-1H-吲唑
CAS:29110-74-5 |
|
 |
4-吡唑甲酸乙酯
CAS:37622-90-5 |