Solution structures of 1:1 complexes of oxyphenonium bromide with beta- and gamma-cyclodextrins.
Noriaki Funasaki, Tomoko Sumiyoshi, Seiji Ishikawa, Saburo Neya
文献索引:Mol. Pharm. 1(2) , 166-72, (2004)
全文:HTML全文
摘要
The solution structures of complexes of oxyphenonium bromide (OB) with beta- and gamma-cyclodextrins (beta- and gamma-CDs, respectively) in deuterium oxide have been investigated by 500 MHz proton NMR spectroscopy and molecular mechanics calculations. The chemical shifts induced by complex formation provide the 1:1 binding constants and the chemical shift variations, DeltadeltaOB-CD, with complexation for the protons of OB and the CDs. The observed binding constants are very close to those obtained by other methods and are in the following order: beta-CD > gamma-CD > alpha-CD. Initial structures of the complexes are constructed on the basis of the ROESY spectra and the DeltadeltaOB-CD values and are optimized by molecular mechanics calculations. The intermolecular distances between the protons of OB and CD calculated for these structures are well-correlated with the observed ROESY intensities. The cyclohexyl group of OB penetrates deeply into a beta-CD cavity, and the phenyl group is close to the wide rim of the cavity. The phenyl and cyclohexyl groups of OB are both incorporated into a gamma-CD cavity. Furthermore, these structures of the complexes are consistent with the suppression of bitter taste and basic hydrolysis of OB by CDs and the polarity of binding sites of OB.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
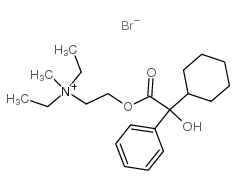 |
奥芬溴铵
CAS:50-10-2 |
C21H34BrNO3 |
|
Ultraviolet spectroscopic estimation of microenvironments an...
1999-08-01 [J. Pharm. Sci. 88(8) , 759-62, (1999)] |
|
Electron paramagnetic resonance and electron nuclear double ...
2000-04-11 [Biochemistry 39(14) , 4112-21, (2000)] |
|
Safety pharmacology of a combination of tinidazole and oxyph...
1997-07-01 [Arzneimittelforschung 47(7) , 869-72, (1997)] |
|
Stability-indicating method for determination of oxyphenoniu...
2007-01-01 [J. AOAC Int. 90(5) , 1250-7, (2007)] |
|
Cholinergic modulation of anaphylactic shock: plasma protein...
2007-05-30 [Life Sci. 80(24-25) , 2342-6, (2007)] |