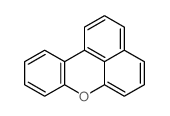
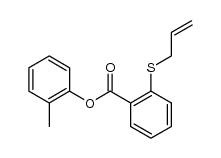
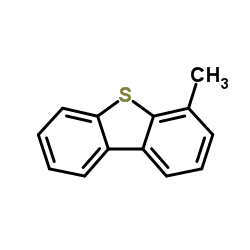
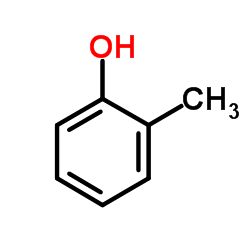
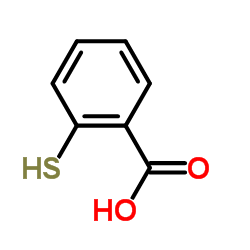
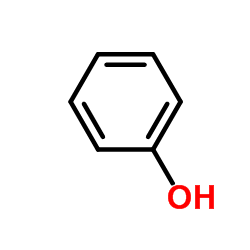
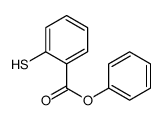
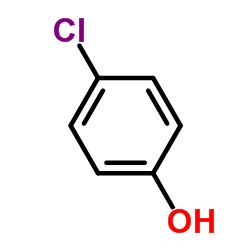
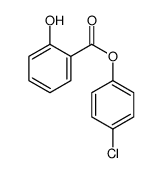
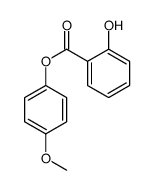
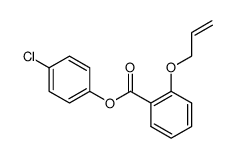
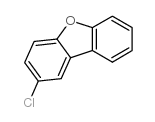
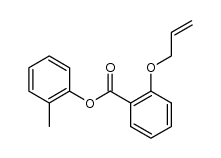
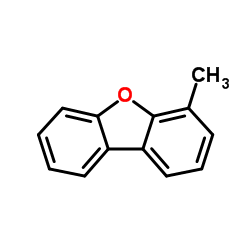
|
~% |
|
~% |
|
~% |
|
~40% |
|
~58% |
|
~% |
|
~% |
|
~72% |
|
~92% |
|
~% |
|
~% |
|
~92% |
|
~30% |
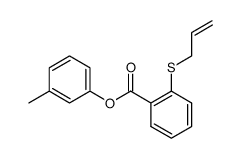
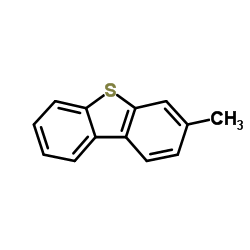
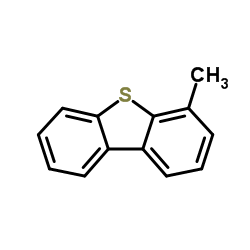
![4-Methyldibenzo[b,d]thiophene结构式](https://image.chemsrc.com/caspic/164/31317-07-4.png)
![苯并[b]萘并[2,1-d]呋喃结构式](https://image.chemsrc.com/caspic/274/239-30-5.png)