| 结构式 | 名称/CAS号 | 全部文献 |
|---|---|---|
 |
三氟甲磺酸锂
CAS:33454-82-9 |
|
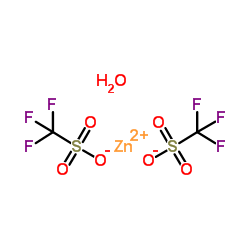 |
三氟甲烷磺酸锌
CAS:54010-75-2 |
|
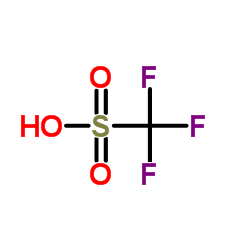 |
三氟甲烷磺酸
CAS:1493-13-6 |
|
 |
三氟甲磺酸钡
CAS:2794-60-7 |