| 结构式 | 名称/CAS号 | 全部文献 |
|---|---|---|
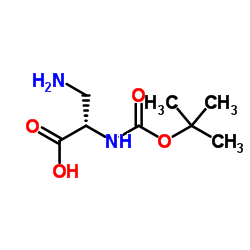 |
N(α)-Boc-L-2,3-二氨丙酸
CAS:73259-81-1 |
|
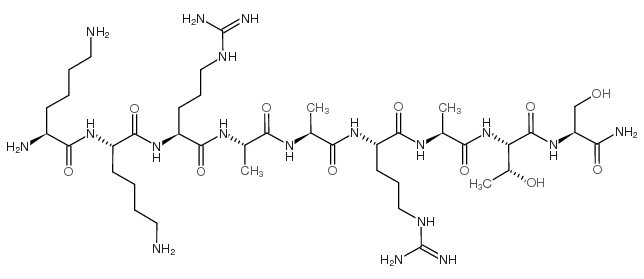 |
LYS-LYS-ARG-ALA-ALA-ARG-ALA-THR-SER-NH2
CAS:119386-39-9 |