Synthesis and evaluation of heteroaryl-ketone derivatives as a novel class of VEGFR-2 inhibitors.
Eugene L Piatnitski Chekler, Reeti Katoch-Rouse, Alexander S Kiselyov, Dan Sherman, Xiaohu Ouyang, Ki Kim, Ying Wang, Yaron R Hadari, Jacqueline F Doody, Eugene L Piatnitski Chekler, Reeti Katoch-Rouse, Alexander S Kiselyov, Dan Sherman, Xiaohu Ouyang, Ki Kim, Ying Wang, Yaron R Hadari, Jacqueline F Doody, Eugene L. Piatnitski Chekler, Reeti Katoch-Rouse, Alexander S. Kiselyov, Dan Sherman, Xiaohu Ouyang, Ki Kim, Ying Wang, Yaron R. Hadari, Jacqueline F. Doody
文献索引:Bioorg. Med. Chem. Lett. 18 , 4344, (2008)
全文:HTML全文
摘要
We have discovered novel inhibitors of VEGFR-2 kinase with low nanomolar potency in both enzymatic and cell-based assays. Active series are heteroaryl-ketone compounds containing a central aromatic ring with either an indazolyl or indolyl keto group in the ortho orientation to the benzylic amine group (Fig. 1). The best compounds were demonstrated to be inactive against a small select panel of tyrosine and serine/threonine kinases with the exception of VEGFR-1 kinase, a close family member. In addition, the lead candidate 8 displayed acceptable exposure levels when administered orally to mice.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
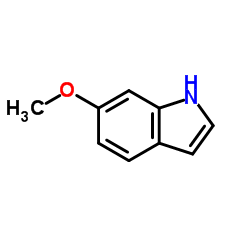 |
6-甲氧基吲哚
CAS:3189-13-7 |
C9H9NO |
|
Tryptophan 2,3-dioxygenase (TDO) inhibitors. 3-(2-(pyridyl)e...
2011-08-11 [J. Med. Chem. 54 , 5320, (2011)] |
|
Discovery of a novel class of PPARdelta partial agonists.
2008-09-15 [Bioorg. Med. Chem. Lett. 18 , 5018, (2008)] |
|
Synthesis and evaluation of 3-aroylindoles as anticancer age...
2009-08-13 [J. Med. Chem. 52 , 4941, (2009)] |
|
Synthesis of 3,3-diindolyl oxyindoles efficiently catalysed ...
2010-09-01 [Bioorg. Med. Chem. Lett. 20 , 5229, (2010)] |
|
Indole ring oxidation by activated leukocytes prevents the p...
2005-11-01 [Braz. J. Med. Biol. Res. 38(11) , 1575-83, (2005)] |