An efficient synthesis of 2-substituted 6-methylpurine bases and nucleosides by Fe- or Pd-catalyzed cross-coupling reactions of 2,6-dichloropurines.
Michal Hocek, Hana Dvoráková
文献索引:J. Org. Chem. 68(14) , 5773-6, (2003)
全文:HTML全文
摘要
Fe-catalyzed cross-coupling reactions of 9-substituted or protected 2,6-dichloropurines with 1 equiv of methylmagnesium chloride gave regioselectively 2-chloro-6-methylpurines in good yields. The same reactions with 3 equiv of methylmagnesium chloride or Pd-catalyzed reactions with trimethylaluminum afforded 2,6-dimethylpurines. The 2-chloro-6-methylpurines underwent another coupling with phenylboronic acid to give 6-methyl-2-phenylpurines. All reactions were perfomed for Bn- and THP-protected purine bases as well as for acyl-protected ribosides and 2-deoxyribosides. After deprotection, free purine bases and nucleosides were obtained.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
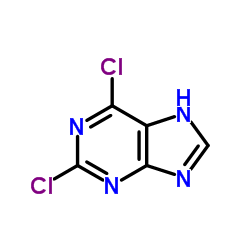 |
2,6-二氯嘌呤
CAS:5451-40-1 |
C5H2Cl2N4 |
|
Occupational contact sensitization to 2,6-dichloropurine.
1981-11-01 [Contact Dermatitis 7(6) , 349-50, (1981)] |
|
Occupational contact sensitization to 2,6-dichloropurine.
1981-05-01 [Contact Dermatitis 7(3) , 162-3, (1981)] |
|
Two tautomeric polymorphs of 2,6-dichloropurine.
2011-12-01 [Acta Crystallogr. C 67(Pt 12) , o484-6, (2011)] |
|
Method for the synthesis of uric acid derivatives.
2000-07-01 [Nucleosides Nucleotides Nucleic Acids 19(7) , 1193-203, (2000)] |
|
A class of novel conjugates of substituted purine and Gly-AA...
2010-10-15 [Bioorg. Med. Chem. Lett. 20 , 6157-60, (2010)] |