| 结构式 | 名称/CAS号 | 全部文献 |
|---|---|---|
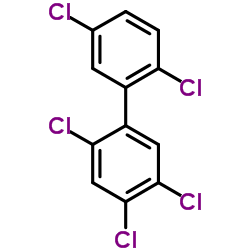 |
2,2,4,5,5-五氯联苯
CAS:37680-73-2 |
|
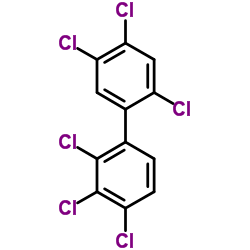 |
异辛烷中PCB138溶液
CAS:35065-28-2 |