| 结构式 | 名称/CAS号 | 全部文献 |
|---|---|---|
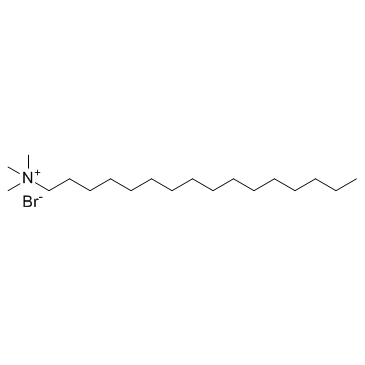 |
十六烷基三甲基溴化铵
CAS:57-09-0 |
|
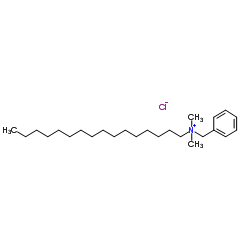 |
十六烷基二甲基苄基氯化铵
CAS:122-18-9 |
|
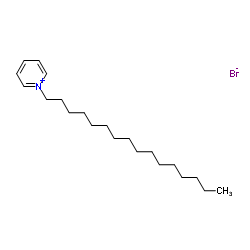 |
溴代十六烷基吡啶
CAS:140-72-7 |