Purification and characterization of cellobiose dehydrogenase from the plant pathogen Sclerotium (Athelia) rolfsii.
U Baminger, S S Subramaniam, V Renganathan, D Haltrich
文献索引:Appl. Environ. Microbiol. 67 , 1766-1774, (2001)
全文:HTML全文
摘要
Cellobiose dehydrogenase (CDH) is an extracellular hemoflavoenzyme produced by several wood-degrading fungi. In the presence of a suitable electron acceptor, e.g., 2,6-dichloro-indophenol (DCIP), cytochrome c, or metal ions, CDH oxidizes cellobiose to cellobionolactone. The phytopathogenic fungus Sclerotium rolfsii (teleomorph: Athelia rolfsii) strain CBS 191.62 produces remarkably high levels of CDH activity when grown on a cellulose-containing medium. Of the 7,500 U of extracellular enzyme activity formed per liter, less than 10% can be attributed to the proteolytic product cellobiose:quinone oxidoreductase. As with CDH from wood-rotting fungi, the intact, monomeric enzyme from S. rolfsii contains one heme b and one flavin adenine dinucleotide cofactor per molecule. It has a molecular size of 101 kDa, of which 15% is glycosylation, and a pI value of 4.2. The preferred substrates are cellobiose and cellooligosaccharides; additionally, beta-lactose, thiocellobiose, and xylobiose are efficiently oxidized. Cytochrome c (equine) and the azino-di-(3-ethyl-benzthiazolin-6-sulfonic acid) cation radical were the best electron acceptors, while DCIP, 1,4-benzoquinone, phenothiazine dyes such as methylene blue, phenoxazine dyes such as Meldola's blue, and ferricyanide were also excellent acceptors. In addition, electrons can be transferred to oxygen. Limited in vitro proteolysis with papain resulted in the formation of several protein fragments that are active with DCIP but not with cytochrome c. Such a flavin-containing fragment, with a mass of 75 kDa and a pI of 5.1 and lacking the heme domain, was isolated and partially characterized.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
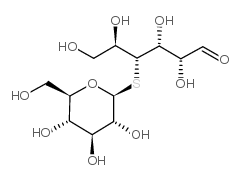 |
硫代纤维二糖
CAS:80951-92-4 |
C12H22O10S |
|
Substrate specificity of cellobiose dehydrogenase from Phane...
1998-03-03 [Biochim. Biophys. Acta 1383 , 48-54, (1998)] |
|
NMR studies of the conformation of thiocellobiose bound to a...
1998-01-16 [FEBS Lett. 421 , 243-248, (1998)] |
|
Crystal structures of Paenibacillus polymyxa beta-glucosidas...
2007-08-31 [J. Mol. Biol. 371 , 1204-1218, (2007)] |
|
Induction of cellulose in Schizophyllum commune: thiocellobi...
1982-01-01 [J. Bacteriol. 149 , 47, (1982)] |
|
Alleviating product inhibition in cellulase enzyme Cel7A.
2016-02-01 [Biotechnol. Bioeng. 113 , 330-8, (2016)] |