| 结构式 | 名称/CAS号 | 全部文献 |
|---|---|---|
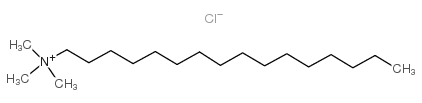 |
十六烷基三甲基氯化铵
CAS:112-02-7 |
|
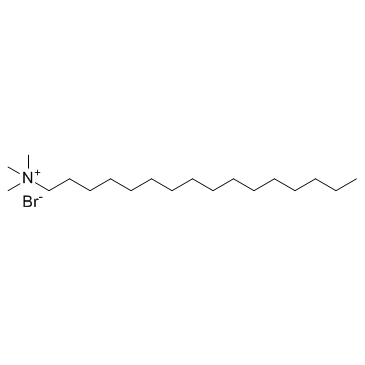 |
十六烷基三甲基溴化铵
CAS:57-09-0 |
|
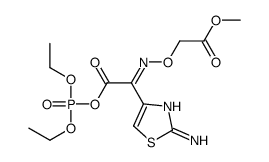 |
等规聚(甲基丙烯酸甲酯)
CAS:25188-98-1 |
|
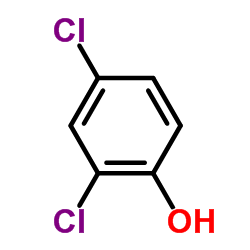 |
2,4-二氯苯酚
CAS:120-83-2 |
|
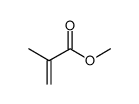 |
聚(甲基丙烯酸甲酯)
CAS:9011-14-7 |
|
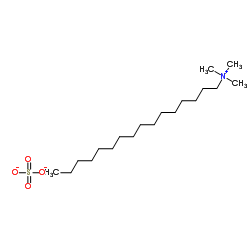 |
十六烷基三甲基硫酸氢铵
CAS:68214-07-3 |