Journal of Organic Chemistry
2007-04-13
Stereoselective synthesis of di- and monofluoromethylated vicinal ethylenediamines with di- and monofluoromethyl sulfones.
Jun Liu, Ya Li, Jinbo Hu
文献索引:J. Org. Chem. 8th ed., 72 , 3119-3121, (2007)
全文:HTML全文
摘要
The diastereoselective nucleophilic (phenylsulfonyl)difluoromethylation and (phenylsulfonyl)monofluoromethylation of alpha-amino N-tert-butanesulfinimines (3) by using PhSO2CF2H and PhSO2CH2F reagents gave products 4 or 5 in high yields (73-99%) and with excellent diastereoselectivity (dr up to >99:1). After subsequent reductive desulfonylation and acid-catalyzed alcoholysis, compounds 4 and 5 could be readily transformed to chiral alpha-difluoromethylated or alpha-monofluoromethylated ethylenediamines in good yields.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
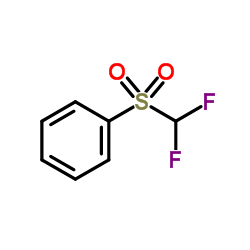 |
二氟甲基苯基砜
CAS:1535-65-5 |
C7H6F2O2S |
相关文献:
更多...
|
Nucleophilic fluoroalkylation of alpha,beta-enones, arynes, ...
2008-08-01 [J. Org. Chem. 15th ed., 73 , 5699-5713, (2008)] |
|
Preparation of tri- and difluoromethylsilanes via an unusual...
2003-05-30 [J. Org. Chem. 11th ed., 68 , 4457-4463, (2003)] |
|
Facile synthesis of chiral alpha-difluoromethyl amines from ...
2005-09-12 [Angew. Chem. Int. Ed. Engl. 36th ed., 44 , 5882-5886, (2005)] |
|
Nucleophilic difluoromethylation of primary alkyl halides us...
2004-11-11 [Org. Lett. 23th ed., 6 , 4315-4317, (2004)] |
|
A remarkably efficient fluoroalkylation of cyclic sulfates a...
2007-01-01 [Angew. Chem. Int. Ed. Engl. 5th ed., 46 , 786-789, (2007)] |