| 结构式 | 名称/CAS号 | 全部文献 |
|---|---|---|
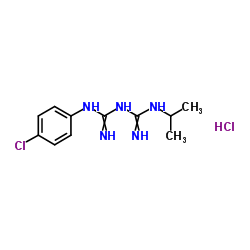 |
吉非罗齐杂质A
CAS:637-32-1 |
|
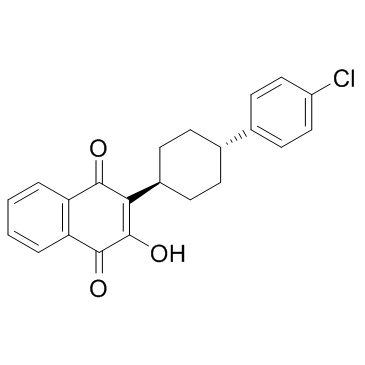 |
阿托伐醌
CAS:95233-18-4 |
| 结构式 | 名称/CAS号 | 全部文献 |
|---|---|---|
 |
吉非罗齐杂质A
CAS:637-32-1 |
|
 |
阿托伐醌
CAS:95233-18-4 |