| 结构式 | 名称/CAS号 | 全部文献 |
|---|---|---|
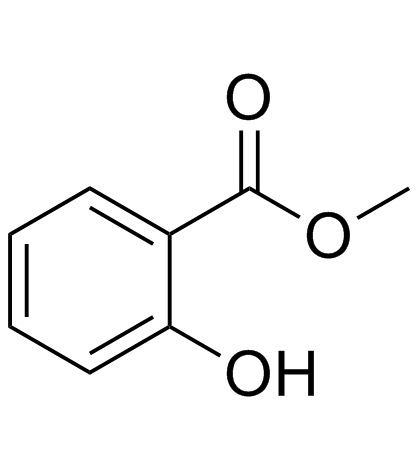 |
水杨酸甲酯
CAS:119-36-8 |
|
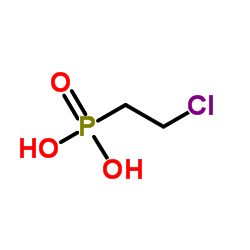 |
乙烯利
CAS:16672-87-0 |
| 结构式 | 名称/CAS号 | 全部文献 |
|---|---|---|
 |
水杨酸甲酯
CAS:119-36-8 |
|
 |
乙烯利
CAS:16672-87-0 |