Efficient synthesis of (+)-1,8,8a-tri-epi-swainsonine, (+)-1,2-di-epi-lentiginosine, (+)-9a-epi-homocastanospermine and (-)-9-deoxy-9a-epi-homocastanospermine from a D-glucose-derived aziridine carboxylate, and study of their glycosidase inhibitory activities.
K S Ajish Kumar, Vinod D Chaudhari, Dilip D Dhavale
文献索引:Org. Biomol. Chem. 6(4) , 703-11, (2008)
全文:HTML全文
摘要
The utility of a D-glucose-derived aziridine carboxylate was demonstrated for the synthesis of polyhydroxylated quinolizidine and indolizidine alkaloids. The chemoselective reduction of 1 followed by two-carbon homologation by the Wittig reaction afforded gamma,delta-aziridino-alpha,beta-unsaturated ester 9, which on regioselective nucleophilic aziridine ring opening either by using water as a nucleophile or hydrogenation afforded delta-lactams 11/16--true synthons for the synthesis of four structurally different iminosugars, namely quinolizidine alkaloids 5b/5c, swainsonine 6b and lentiginosine 7b analogues. Glycosidase inhibitory activities of these iminosugars were investigated.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
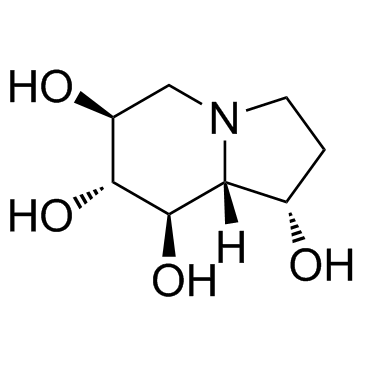 |
栗精胺
CAS:79831-76-8 |
C8H15NO4 |
|
LMAN1 (ERGIC-53) is a potential carrier protein for matrix m...
2015-08-28 [Biochem. Biophys. Res. Commun. 464 , 685-91, (2015)] |
|
Evolution of polyphenols and organic acids during the fermen...
2014-11-01 [Biochem. J. 469(1) , 83-95, (2015)] |
|
Inhibitors of the tick-borne, hemorrhagic fever-associated f...
2014-06-01 [Antimicrob. Agents Chemother. 58(6) , 3206-16, (2014)] |
|
Tetrapisispora phaffii killer toxin is a highly specific bet...
2009-01-01 [Microb. Cell Fact. 8 , 55, (2009)] |
|
Inhibition of the glucosyltransferase activity of clostridia...
2008-06-25 [FEBS Lett. 582(15) , 2277-82, (2008)] |