The action of bromoconduritol on ER glucosidase II.
Yoichi Takeda, Kiichiro Totani, Ichiro Matsuo, Yukishige Ito
文献索引:Bioorg. Med. Chem. Lett. 20 , 5357-9, (2010)
全文:HTML全文
摘要
Bromoconduritol (6-bromo-3,4,5-trihydroxycyclohex-1-ene; BCD) has been known as an inhibitor of glucosidase II (G-II), which plays pivotal roles in glycoprotein processing and folding in the ER. Previous works suggested that BCD specifically inhibits the cleavage of the innermost glucose (Glc) among two alpha1-3 linked Glc residues (cleavage-2). This study addressed the mode of BCD's inhibition toward G-II by using fluorescently labeled substrates. Our analysis clarified that BCD inhibits both cleavage-1 and cleavage-2 activities of G-II. However, the inhibitory activity toward cleavage-2 was 6-fold higher than that toward cleavage-1. Inhibition against both of these activities was retained after dialysis, supporting that BCD exhibits inhibition through irreversible binding to G-II.Copyright 2009. Published by Elsevier Ltd.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
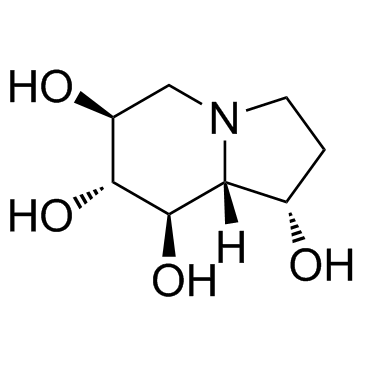 |
栗精胺
CAS:79831-76-8 |
C8H15NO4 |
|
LMAN1 (ERGIC-53) is a potential carrier protein for matrix m...
2015-08-28 [Biochem. Biophys. Res. Commun. 464 , 685-91, (2015)] |
|
Evolution of polyphenols and organic acids during the fermen...
2014-11-01 [Biochem. J. 469(1) , 83-95, (2015)] |
|
Inhibitors of the tick-borne, hemorrhagic fever-associated f...
2014-06-01 [Antimicrob. Agents Chemother. 58(6) , 3206-16, (2014)] |
|
Tetrapisispora phaffii killer toxin is a highly specific bet...
2009-01-01 [Microb. Cell Fact. 8 , 55, (2009)] |
|
Inhibition of the glucosyltransferase activity of clostridia...
2008-06-25 [FEBS Lett. 582(15) , 2277-82, (2008)] |