Cancer-associated fibroblast-targeted strategy enhances antitumor immune responses in dendritic cell-based vaccine.
Yasuhiko Ohshio, Koji Teramoto, Jun Hanaoka, Noriaki Tezuka, Yasushi Itoh, Tohru Asai, Yataro Daigo, Kazumasa Ogasawara
文献索引:Cancer Sci. 106(2) , 134-42, (2015)
全文:HTML全文
摘要
Given the close interaction between tumor cells and stromal cells in the tumor microenvironment (TME), TME-targeted strategies would be promising for developing integrated cancer immunotherapy. Cancer-associated fibroblasts (CAFs) are the dominant stromal component, playing critical roles in generation of the pro-tumorigenic TME. We focused on the immunosuppressive trait of CAFs, and systematically explored the alteration of tumor-associated immune responses by CAF-targeted therapy. C57BL/6 mice s.c. bearing syngeneic E.G7 lymphoma, LLC1 Lewis lung cancer, or B16F1 melanoma were treated with an anti-fibrotic agent, tranilast, to inhibit CAF function. The infiltration of immune suppressor cell types, including regulatory T cells and myeloid-derived suppressor cells, in the TME was effectively decreased through reduction of stromal cell-derived factor-1, prostaglandin E2 , and transforming growth factor-β. In tumor-draining lymph nodes, these immune suppressor cell types were significantly decreased, leading to activation of tumor-associated antigen-specific CD8(+) T cells. In addition, CAF-targeted therapy synergistically enhanced multiple types of systemic antitumor immune responses such as the cytotoxic CD8(+) T cell response, natural killer activity, and antitumor humoral immunity in combination with dendritic cell-based vaccines; however, the suppressive effect on tumor growth was not observed in tumor-bearing SCID mice. These data indicate that systemic antitumor immune responses by various immunologic cell types are required to bring out the efficacy of CAF-targeted therapy, and these effects are enhanced when combined with effector-stimulatory immunotherapy such as dendritic cell-based vaccines. Our mouse model provides a novel rationale with TME-targeted strategy for the development of cell-based cancer immunotherapy. © 2014 The Authors. Cancer Science published by Wiley Publishing Asia Pty Ltd on behalf of Japanese Cancer Association.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
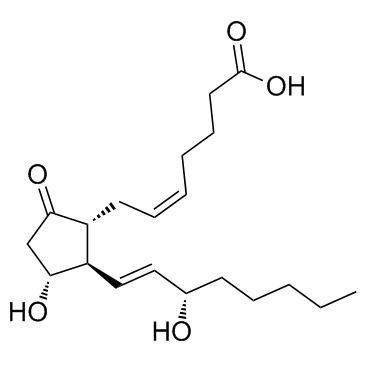 |
地诺前列酮
CAS:363-24-6 |
C20H32O5 | |
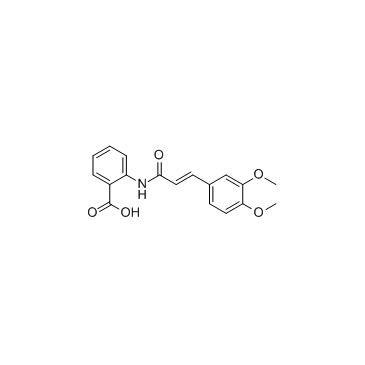 |
曲尼司特
CAS:53902-12-8 |
C18H17NO5 |
|
MLN4924 sensitizes monocytes and maturing dendritic cells fo...
2015-03-01 [Br. J. Pharmacol. 172(5) , 1222-36, (2015)] |
|
Isothiocyanate analogs targeting CD44 receptor as an effecti...
2014-07-01 [Med. Chem. Res. 23(8) , 3836-3851, (2014)] |
|
Unique characteristics of human mesenchymal stromal/progenit...
2014-11-01 [Cytotherapy 16(11) , 1486-500, (2014)] |
|
HuR mediates the synergistic effects of angiotensin II and I...
2015-06-01 [Br. J. Pharmacol. 172 , 3028-42, (2015)] |
|
Kaposi's sarcoma-associated herpesvirus induces Nrf2 activat...
2015-02-01 [J. Virol. 89(4) , 2268-86, (2015)] |