| 结构式 | 名称/CAS号 | 全部文献 |
|---|---|---|
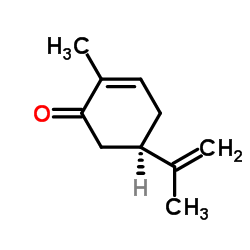 |
左旋香芹酮
CAS:6485-40-1 |
|
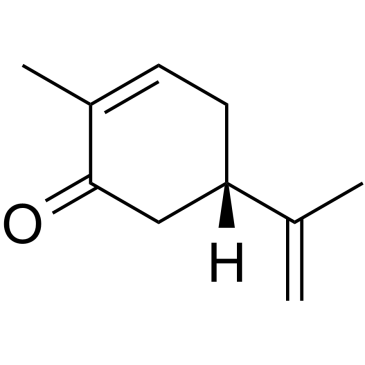 |
(S)-(+)-香芹酮
CAS:2244-16-8 |
| 结构式 | 名称/CAS号 | 全部文献 |
|---|---|---|
 |
左旋香芹酮
CAS:6485-40-1 |
|
 |
(S)-(+)-香芹酮
CAS:2244-16-8 |