Intramolecular charge transfer and dielectric solvent relaxation in n-propyl cyanide. N-phenylpyrrole and 4-dimethylamino-4'-cyanostilbene.
Sergey I Druzhinin, Victor A Galievsky, Toshitada Yoshihara, Klaas A Zachariasse
文献索引:J. Phys. Chem. A 110(47) , 12760-8, (2006)
全文:HTML全文
摘要
Fast intramolecular charge transfer (ICT) accompanied by dual fluorescence from a locally excited (LE) and an ICT state taking place with N-phenylpyrrole (PP) in the solvent n-propyl cyanide (PrCN) is investigated as a function of temperature between 25 and -112 degrees C. The LE and ICT fluorescence decays from -45 to -70 degrees C can be adequately fitted with two exponentials, in accordance with a two state (LE + ICT) reaction mechanism, similar to what has been observed with PP in the more polar and less viscous alkyl cyanides acetonitrile (MeCN) and ethyl cyanide (EtCN). At lower temperatures, triple-exponential fits are required for the LE and ICT decays. The ICT emission band maximum of the time-resolved fluorescence spectra of PP in PrCN at -100 degrees C displays a spectral shift from 29 230 cm-1 at t = 0 to 27 780 cm-1 at infinite time, which equilibration process is attributed to dielectric solvent relaxation. From the time dependence of this shift, in global analysis with that of the band integrals BI(LE) and BI(ICT) of the time-resolved LE and ICT fluorescence bands, the decay times 119 and 456 ps are obtained. Dielectric relaxation times of 20 and 138 ps are determined from the double-exponential spectral solvation response function C(t) of the probe molecule 4-dimethylamino-4'-cyanostilbene in PrCN at -100 degrees C. It is concluded from the similarity of the times 119 ps (PP) and 138 ps (DCS) that the deviation from double-exponential character for the fluorescence decays of PP in PrCN below -70 degrees C is due to the interference of dielectric solvent relaxation with the ICT reaction. This fact complicates the kinetic analysis of the LE and ICT fluorescence decays. The kinetic analysis for PP in PrCN is hence restricted to temperatures between -70 and -45 degrees C. From this analysis, the forward and backward ICT activation energies Ea (12 kJ/mol) and Ed (17 kJ/mol) are obtained, giving an ICT stabilization enthalpy -DeltaH of 5 kJ/mol. A comparison of the reaction barriers for PP in the three alkyl cyanides PrCN, EtCN, and MeCN (J. Phys. Chem. A 2005, 109, 1497) shows that Ea becomes smaller with increasing solvent polarity (from 12 to 6 kJ/mol), whereas Ed remains effectively constant. Both observations are indicative of a late transition state for the LE --> ICT reaction. The significance of the Leffler-Hammond postulate in this connection is discussed: not primarily the energy of the LE, ICT, and transition states but rather the extent of charge transfer in these states determines whether an early or a late transition state is present.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
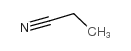 |
丙腈
CAS:107-12-0 |
C3H5N |
|
Functionalization of graphene with self-doped conducting pol...
2015-10-01 [J. Colloid. Interface Sci. 455 , 63-70, (2015)] |
|
G protein-coupled estrogen receptor (GPER) mediates NSCLC pr...
2015-04-01 [Med. Oncol. 32(4) , 104, (2015)] |
|
In vitro metabolic conversion of the organic breakdown produ...
2015-08-30 [J. Sci. Food Agric. 95 , 2244-51, (2015)] |
|
High IL-17E and low IL-17C dermal expression identifies a fi...
2014-01-01 [PLoS ONE 9(8) , e105008, (2014)] |
|
Critical period for dopaminergic neuroprotection by hormonal...
2015-02-01 [Neurobiol. Aging 36(2) , 1194-208, (2015)] |