| 结构式 | 名称/CAS号 | 全部文献 |
|---|---|---|
 |
曙红Y(水溶)
CAS:17372-87-1 |
|
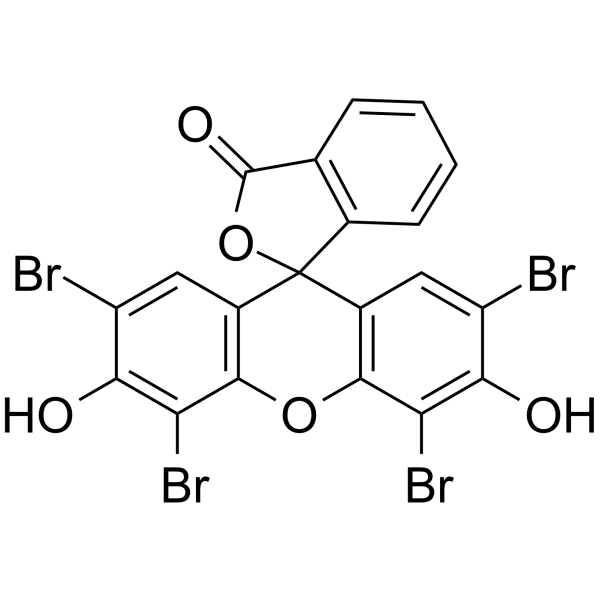 |
曙红Y(醇溶)
CAS:15086-94-9 |
|
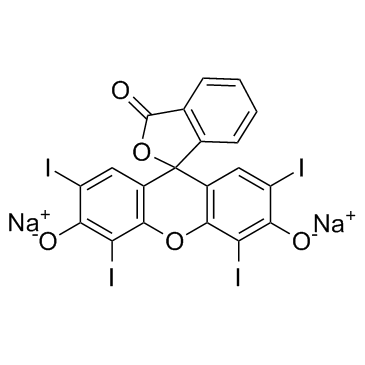 |
赤藓红B
CAS:16423-68-0 |