Cloning and expression of beta-glucuronidase from Lactobacillus brevis in E. coli and application in the bioconversion of baicalin and wogonoside.
Hyun Sung Kim, Jin Yong Kim, Myeong Soo Park, Hua Zheng, Geun Eog Ji
文献索引:J. Microbiol. Biotechnol. 19(12) , 1650-5, (2009)
全文:HTML全文
摘要
The beta-glucuronidase (GUS) gene from Lactobacillus brevis RO1 was cloned and expressed in Escherichia coli GMS407. The GUS gene was composed of 1812 bp, encoding a 603-amino-acid protein belonging to the glycosyl hydrolase family 2 with three conserved domains. The amino acid similarity was higher than 70% with the beta-glucuronidases of various microorganisms, yet less than 58% with the beta-glucuronidase of L. gasseri ADH. Overexpression and purification of the GUS was performed in beta-glucuronidase-deficient E. coli GMS407. The purified GUS protein was 71 kDa and showed 1284 U/mg of specific activity at optimum condition of pH 5.0 and 37 degrees C. At 37 degrees C, the GUS remained stable for 80 min at pH values ranging from 5.0 to 8.0. The purified enzyme exhibited a half-life of 1 h at 60 degrees C and more than 2 h at 50 degrees C. When the purified GUS was applied to transform baicalin and wogonoside into their corresponding aglycones, 150 microM of baicalin and 125 microM of wogonoside were completely transformed into baicalein and wogonin, respectively, within 3 h.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
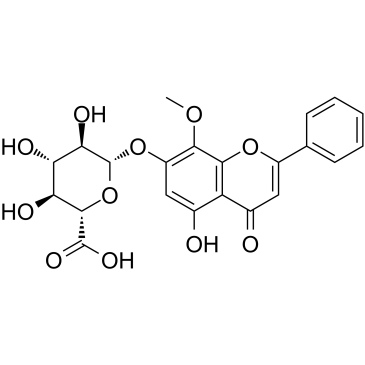 |
汉黄芩苷
CAS:51059-44-0 |
C22H20O11 |
|
Extraction and Bioactivity Analysis of Major Flavones Compou...
2014-01-01 [J. Anal. Methods Chem. 2014 , 563702, (2014)] |
|
Wogonoside induces autophagy-related apoptosis in human glio...
2014-09-01 [Oncol. Rep. 32(3) , 1179-87, (2014)] |
|
Liquid Chromatography-Tandem Mass Spectrometry Screening Met...
2015-08-01 [J. Chromatogr. Sci. 53 , 1140-6, (2015)] |
|
Simultaneous determination of various bioactive redox compon...
2010-01-01 [J. Pharm. Biomed. Anal. 95 , 93-101, (2014)] |
|
Studies on the antioxidant properties of extracts from the r...
2015-01-01 [Acta Biochim. Pol. 62 , 253-8, (2015)] |