| 结构式 | 名称/CAS号 | 全部文献 |
|---|---|---|
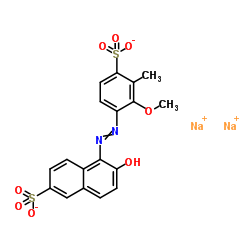 |
食品红17
CAS:25956-17-6 |
|
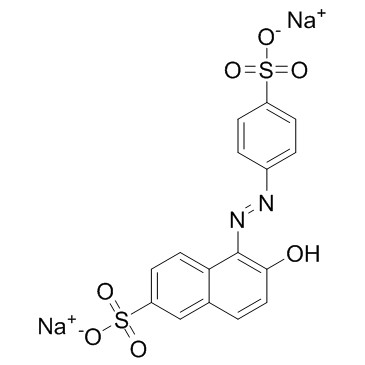 |
日落黄
CAS:2783-94-0 |
| 结构式 | 名称/CAS号 | 全部文献 |
|---|---|---|
 |
食品红17
CAS:25956-17-6 |
|
 |
日落黄
CAS:2783-94-0 |