Stabilization of anionic and neutral forms of a fluorophoric ligand at the active site of human carbonic anhydrase I.
Sumathra Manokaran, Jayati Banerjee, Sanku Mallik, D K Srivastava
文献索引:Biochim. Biophys. Acta 1804(10) , 1965-1973, (2010)
全文:HTML全文
摘要
We synthesized a fluorogenic dansylamide derivative (JB2-48), which fills the entire (15 A deep) active site pocket of human carbonic anhydrase I, and investigated the contributions of sulfonamide and hydrophobic regions of the ligand structure on the spectral, kinetic, and thermodynamic properties of the enzyme-ligand complex. The steady-state and fluorescence lifetime data revealed that the deprotonation of the sulfonamide moiety of the enzyme bound ligand increases the fluorescence emission intensity as well as the lifetime of the fluorophores. This is manifested via the electrostatic interaction between the active site resident Zn²+ cofactor and the negatively charged sulfonamide group of the ligand, and such interaction contributes to about 2.2 kcal/mol (ΔΔG°) and 0.89 kcal/mol (ΔΔG(#)) energy in stabilizing the ground and the putative transition states, respectively. We provide evidence that the anionic and neutral forms of JB2-48 are stabilized by the complementary microscopic/conformational states of the enzyme. The implication of the mechanistic studies presented herein in rationale design of carbonic anhydrase inhibitors is discussed.Copyright © 2010 Elsevier B.V. All rights reserved.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
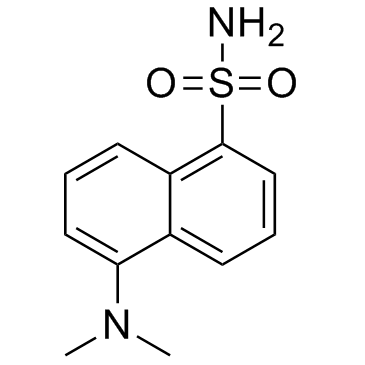 |
丹酰胺
CAS:1431-39-6 |
C12H14N2O2S |
|
Macromolecular Systems with MSA-Capped CdTe and CdTe/ZnS Cor...
2015-11-11 [ACS Appl. Mater. Interfaces 7 , 24778-90, (2015)] |
|
Alteration of human serum albumin tertiary structure induced...
2016-01-15 [Spectrochim. Acta. A. Mol. Biomol. Spectrosc. 153 , 560-5, (2015)] |
|
Optimizing Multiple Analyte Injections in Surface Plasmon Re...
2015-01-01 [Sci. Rep. 5 , 15855, (2015)] |
|
Nutritional and health-promoting properties of bean paste fo...
2015-11-01 [Food Funct. 6 , 3560-6, (2015)] |
|
How many antimicrobial peptide molecules kill a bacterium? T...
2014-09-19 [ACS Chem. Biol. 9(9) , 2003-7, (2014)] |