A fluorescence study of human serum albumin binding sites modification by hypochlorite.
Eduardo Lissi, M Alicia Biasutti, Elsa Abuin, Luis León, Eduardo Lissi, M. Alicia Biasutti, Elsa Abuin, Luis León
文献索引:J. Photochem. Photobiol. B, Biol. 94(2) , 77-81, (2009)
全文:HTML全文
摘要
A study has been made on the properties of human serum albumin (HSA) binding sites and how they are modified by pre-oxidation of the protein with hypochlorite. The oxidation extent was assessed from changes in the protein intrinsic fluorescence and production of carbonyl groups. HSA retains its solute binding capacity even after exposure to relatively large amounts of hypochlorite (up to 40 oxidant molecules per protein). From an analysis of the binding isotherms of dansyl sarcosine (DS) and dansyl-1-sulfonamide (DNSA) to native and hypochlorite treated albumin it is concluded that pre-oxidation of the protein reduces the number of active sites without affecting the binding capacity of the remaining binding sites. From DS and DNSA fluorescence anisotropy, Laurdan anisotropy and generalized polarization measurements, it is concluded that both Sites I and II in the native protein provide very rigid environments to the bound probes. These characteristics of the sites remain even after extensive treatment with hypochlorite. This stubbornness of HSA could allow the protein to maintain its function along its in vivo lifetime.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
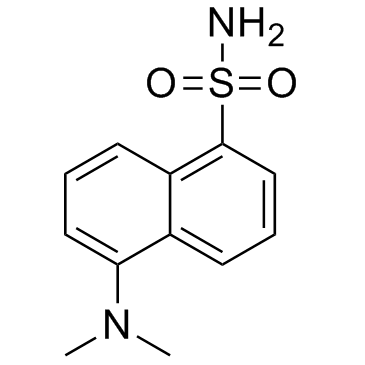 |
丹酰胺
CAS:1431-39-6 |
C12H14N2O2S |
|
Macromolecular Systems with MSA-Capped CdTe and CdTe/ZnS Cor...
2015-11-11 [ACS Appl. Mater. Interfaces 7 , 24778-90, (2015)] |
|
Alteration of human serum albumin tertiary structure induced...
2016-01-15 [Spectrochim. Acta. A. Mol. Biomol. Spectrosc. 153 , 560-5, (2015)] |
|
Optimizing Multiple Analyte Injections in Surface Plasmon Re...
2015-01-01 [Sci. Rep. 5 , 15855, (2015)] |
|
Nutritional and health-promoting properties of bean paste fo...
2015-11-01 [Food Funct. 6 , 3560-6, (2015)] |
|
How many antimicrobial peptide molecules kill a bacterium? T...
2014-09-19 [ACS Chem. Biol. 9(9) , 2003-7, (2014)] |