Carbohydrate binding properties of th Dolichos biflorus lectin and its subunits.
M E Etzler, S Gupta, C Borrebaeck
文献索引:J. Biol. Chem. 256(5) , 2367-70, (1981)
全文:HTML全文
摘要
Equilibrium dialysis studies on the binding of the Dolichos biflorus lectin with [14C]methyl alpha-D-GalNAc showed that the lectin has two combining sites/molecule and an intrinsic association constant at 3 degrees C of 4.2 X 10(3) liters mol-1. Binding studies on individual fractions (II, IV, and VII) of the lectin that differ in their chromatographic properties on concanavalin A-Sepharose gave association constants for methyl alpha-D-GalNAc of 2.2 X 10(3) liters mol-1, 3.2 X 10(3) liters mol-1, and 3.2 X 10(3) liters mol-1, respectively. Molecular exclusion chromatography of iodinated Subunits I and II of the lectin, as well as sedimentation velocity studies of the noniodinated subunits, showed that the isolated subunits form aggregates in aqueous solution. Aggregates of subunit I were capable of agglutinating blood type A erythrocytes, precipitating blood group A + H substance, and binding to blood group A + H substance in an affinity electrophoretic system. Aggregates of subunit II exhibited none of these binding properties and did not inhibit the ability of the intact lectin to agglutinate type A erythrocytes. Affinity electrophoresis of subunit I showed that it has an association constant for N-acetyl-D-galactosamine similar to that of the intact lectin. The results suggest that it is subunit I that is primarily responsible for the carbohydrate binding properties of the lectin.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
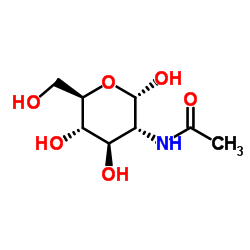 |
甲基 2-乙酰氨基-2-脱氧-α-D-吡喃葡萄糖苷
CAS:6082-04-8 |
C8H15NO6 |
|
Binding of simple carbohydrates and some of their chromophor...
1984-06-10 [J. Biol. Chem. 259(11) , 7067-74, (1984)] |
|
Novel O-linked carbohydrate chains in the cellulase complex ...
1989-01-15 [J. Biol. Chem. 264(2) , 1027-35, (1989)] |
|
Gas--liquid chromatography and mass spectrometry of methylat...
1981-09-15 [Anal. Biochem. 116(2) , 489-502, (1981)] |
|
Selective pivaloylation of 2-acetamido-2-deoxy sugars.
1988-11-01 [Carbohydr. Res. 182(2) , 197-205, (1988)] |
|
Structure of a triphosphonopentaosylceramide containing 4-O-...
1987-10-15 [J. Biol. Chem. 262(29) , 14141-5, (1987)] |