Bioorganic & Medicinal Chemistry
2006-07-01
Synthesis of multisubstituted quinolines from Baylis-Hillman adducts obtained from substituted 2-chloronicotinaldehydes and their antimicrobial activity.
P Narender, U Srinivas, M Ravinder, B Ananda Rao, Ch Ramesh, K Harakishore, B Gangadasu, U S N Murthy, V Jayathirtha Rao
文献索引:Bioorg. Med. Chem. 14(13) , 4600-9, (2006)
全文:HTML全文
摘要
Baylis-Hillman acetates were synthesized from substituted 2-chloronicotinaldehydes and were conveniently transformed into multisubstituted quinolines and cyclopenta[g]quinolines on reaction with nitroethane or ethyl cyanoacetate via a successive S(N)2'-S(N)Ar elimination strategy. Thus, synthesized quinolines were evaluated for antimicrobial activity and found having substantial antibacterial and antifungal activity.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
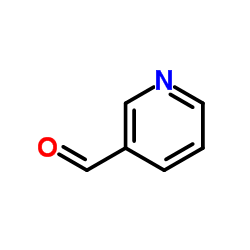 |
吡啶-3-甲醛
CAS:500-22-1 |
C6H5NO |
相关文献:
更多...
|
[Selenazoles. X. [1]. Beta-methyl-beta-4-R-selenazolyl-2)-hy...
1984-01-01 [Ann. Univ. Mariae Curie. Sklodowska. Med. 39 , 33-40, (1984)] |
|
[Selenazoles. IX. [9]. 3-methyl-4-R-selenazolidene-2-hydrazo...
1984-01-01 [Ann. Univ. Mariae Curie. Sklodowska. Med. 39 , 25-31, (1984)] |
|
Kinetic mechanism of sheep liver NADPH-dependent aldehyde re...
1987-02-15 [Biochem. J. 242(1) , 143-50, (1987)] |
|
Subcellular localization of aldehyde reductase activities in...
1981-09-01 [Biochem. J. 197(3) , 715-20, (1981)] |
|
Effect of ozone on nicotine desorption from model surfaces: ...
2006-03-15 [Environ. Sci. Technol. 40(6) , 1799-805, (2006)] |